FDA Approves BOTOX® for Temporary Improvement of Crow’s Feet
September 11, 2013 02:54 PM Eastern Daylight Time
IRVINE, Calif.–(BUSINESS WIRE)–
Allergan, Inc., (NYSE: AGN) today announced approval by the U.S. Food and Drug Administration (FDA) to market BOTOX® Cosmetic (onabotulinumtoxinA), for an additional indication to temporarily treat moderate to severe lateral canthal lines, commonly known as “crow’s feet” lines. BOTOX® Cosmetic is the first and only product of its kind approved for this indication. BOTOX® Cosmetic, approved in the United States in 2002 for the temporary improvement of moderate to severe glabellar lines (frown lines between the brows) for patients aged 18 to 65 years, remains the number-one minimally invasive aesthetic medical treatment globally.
“Allergan has remained the leader in the facial aesthetic industry by prioritizing research and development that provides physicians and patients with innovative treatments and fully explores the additional indications for our product portfolio,” said Scott W. Whitcup M.D., Executive Vice President, Research and Development, Chief Scientific Officer, Allergan. “We are pleased that the FDA has approved a new indication for BOTOX® Cosmetic to temporarily improve the appearance of crow’s feet lines. With this approval, BOTOX® Cosmetic is now the only pharmaceutical approved to treat both crow’s feet lines and frown lines between brows. This approval will enhance our ability to work with and train aesthetic physicians on the science of administering BOTOX® Cosmetic to yield the best possible outcomes for patients.”
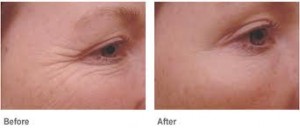
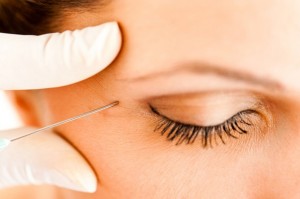 The safety and efficacy of BOTOX® Cosmetic as a treatment for crow’s feet lines was demonstrated in two randomized, multi-center, placebo-controlled clinical trials. The studies enrolled more than 1,350 subjects with 833 subjects receiving treatment with BOTOX® Cosmetic. The trial demonstrated that BOTOX® Cosmetic was an effective treatment compared to the control group, which did not receive BOTOX® Cosmetic treatment.
The safety and efficacy of BOTOX® Cosmetic as a treatment for crow’s feet lines was demonstrated in two randomized, multi-center, placebo-controlled clinical trials. The studies enrolled more than 1,350 subjects with 833 subjects receiving treatment with BOTOX® Cosmetic. The trial demonstrated that BOTOX® Cosmetic was an effective treatment compared to the control group, which did not receive BOTOX® Cosmetic treatment.
“Crow’s feet lines are defined as the lines that extend around the corner of the eye area. They result from years of repetitive squinting and smiling,” said Dr. Steven Dayan, Founder of DeNova Research, Clinical Assistant Professor at the University of Illinois and a clinical investigator in the BOTOX® Cosmetic crow’s feet clinical trials. “I often see patients who are bothered by their crow’s feet lines, so I am very pleased that Allergan has conducted additional research to receive FDA approval of BOTOX® Cosmetic for this new indication. Based on the clinical evidence, I can now provide my patients with an FDA-approved option to address the crow’s feet lines that develop around the eyes.”
 BOTOX® Cosmetic is a prescription medication that is injected into the muscles around the eye area to temporarily improve the look of moderate to severe crow’s feet lines in adults. It is a quick procedure that generally requires no downtime or recovery. BOTOX® Cosmetic works by blocking nerve impulses and reducing movement to the muscles around the eye area. The decreased muscle activity helps lessen the appearance of moderate to severe crow’s feet lines for noticeable results that do not radically change facial appearance or make a patient look as if they have had “work done.”
BOTOX® Cosmetic is a prescription medication that is injected into the muscles around the eye area to temporarily improve the look of moderate to severe crow’s feet lines in adults. It is a quick procedure that generally requires no downtime or recovery. BOTOX® Cosmetic works by blocking nerve impulses and reducing movement to the muscles around the eye area. The decreased muscle activity helps lessen the appearance of moderate to severe crow’s feet lines for noticeable results that do not radically change facial appearance or make a patient look as if they have had “work done.”
BOTOX® Cosmetic should be administered by a licensed, trained healthcare professional. Since 2002, more than 11 million treatment sessions for glabellar lines have been performed with BOTOX® Cosmetic. BOTOX® Cosmetic is approved in more than 75 countries for facial aesthetic use.
To learn more about BOTOX® Cosmetic, visit www.botoxcosmetic.com. Consumers can locate an authorized BOTOX® Cosmetic physician in their area by using the “find a doctor” tool located at www.botoxcosmetic.com.